Hemogam Sagl
Hemogam Sagl is a Swiss company specialized in the human plasma derivatives and biopharmaceuticals.
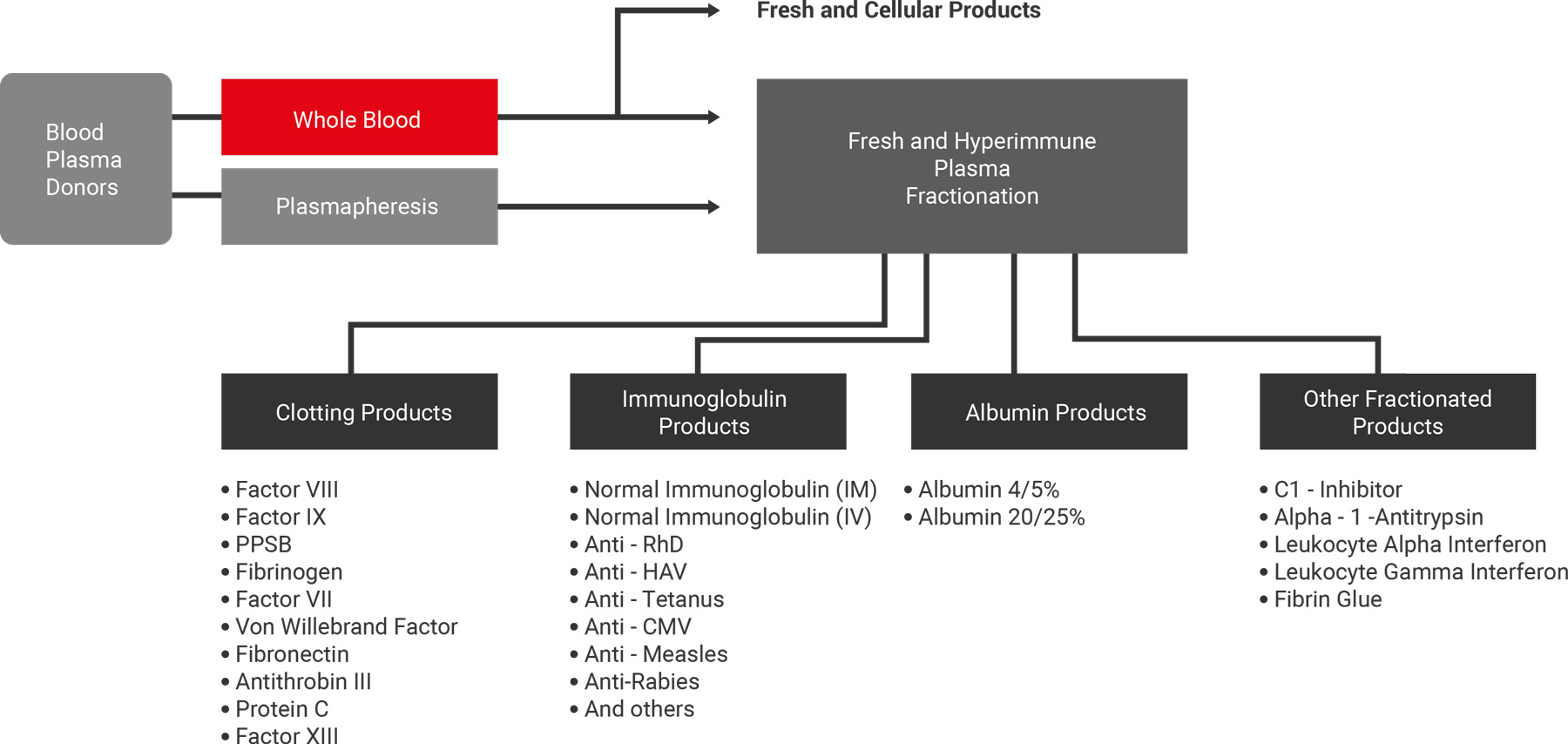
We provide material and technical assistance in the fractioning and other processes to transform human blood into plasma and biopharmaceuticals products.
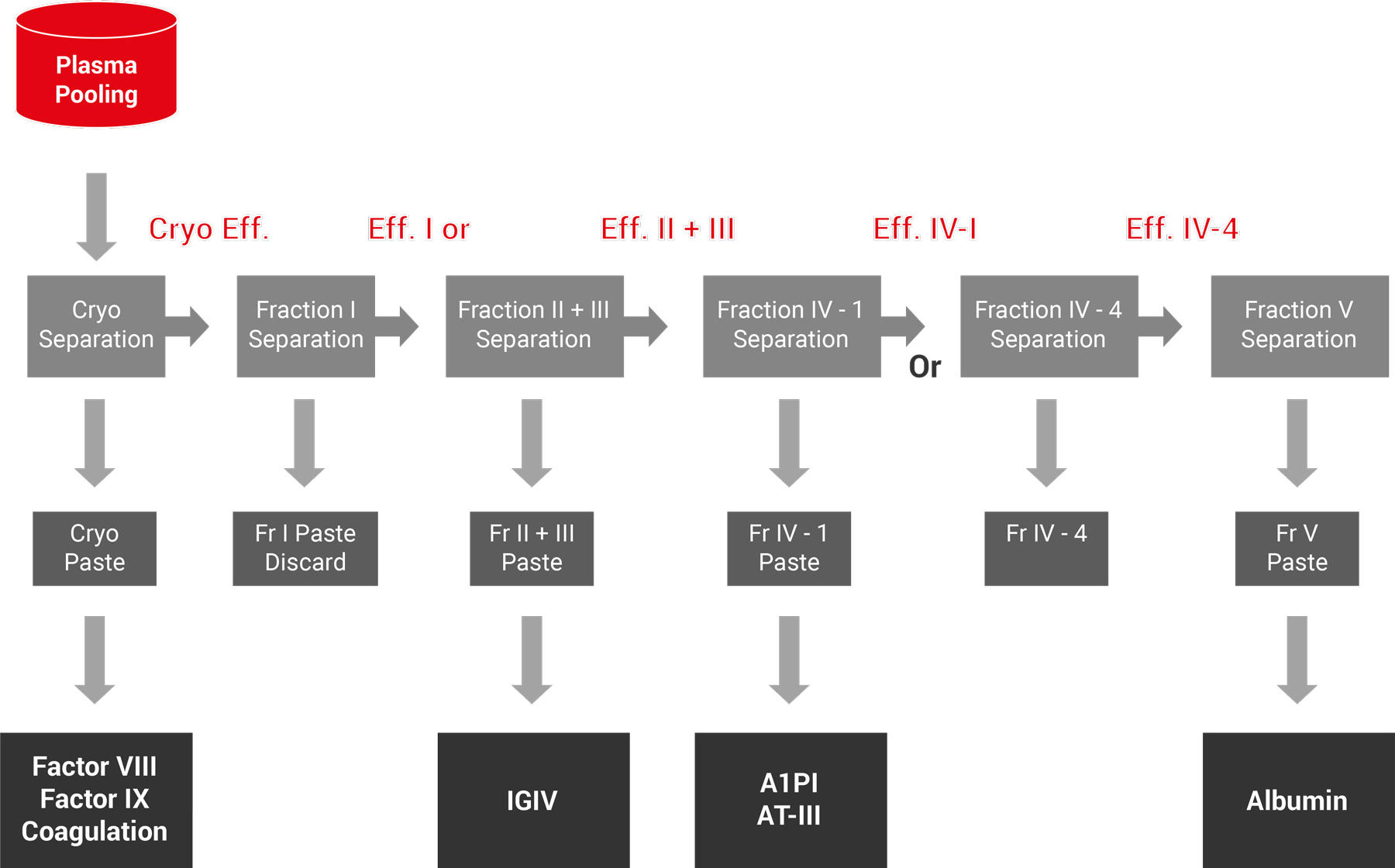
Plasma Fractionation
We provide technical solutions to the human blood plasma fractionation industry, meeting the most stringent requirements of the industry by concomitantly integrating technology, process knowledge and quality systems and training. Our interdisciplinary approach and vast knowledge of the particular features of plasma fractionation make us the preferred partner for fractionation facility/technology projects. Hemogam’s use of modern tools and advanced technologies enable smooth and successful implementation of projects ensuring compliance with Good Manufacturing Practices (GMP).
Integration of the process technology with the automation of plants and operations provide innovative control solutions for each particular process. Repeatability and traceability are of predominant importance, particularly in the plasma derivative industry, where processes must be kept in a validated state. Repeatability is essential as the final product must fulfill strict product specifications. Traceability is crucial when evaluating process performance and determining whether the product has been processed according to the relevant Standard Operating Procedures (SOPs). For companies engaged in plasma fractionation complete traceability is a mandatory regulatory requirement from plasma donors to finished products and from finished products to plasma donors.
Engineering design and process technology of a blood plasma facility is very challenging due to the special features of this unique raw material, human plasma, with inherent risk factors such as viruses, protein denaturation and contamination in combination with complex production processes. Moreover, plasma fractionation is highly regulated with high standards for design and operation of the facility. As a result, product safety, regulatory compliance, traceability and easy operability must all be considered during all design steps. Our approach integrates technology, concepts, process and regulatory knowledge.
Hemogam has accumulated vast experience in plasma fractionation since late 70’s with company principals having more than 120 years of relevant industry experience. Our expertise in innovative processes, technologies and services for protein purification and plasma fractionation combined global capabilities in unmatched.
HEMOGAM Sagl
+41 91 797 45 05
info@hemogam.com
Via Nosedo 1
6900 Lugano-Massagno
Switzerland